Provided that we knows. Mineral Hydrochloric Acid Test Video.

How To Balance Hcl Koh Kcl H2o Hydrochloric Acid Potassium Hydroxide Youtube
Non-carbonate minerals especially silicates will not react to HCl.

. And such a reaction could be performed quantitatively ie. Potassium reacts with dilute hydrochloric acid to give potassium chloride and hydrogen gas. When sodium hydroxide is added to the potassium chromate solution the orange colour turns back to yellow.
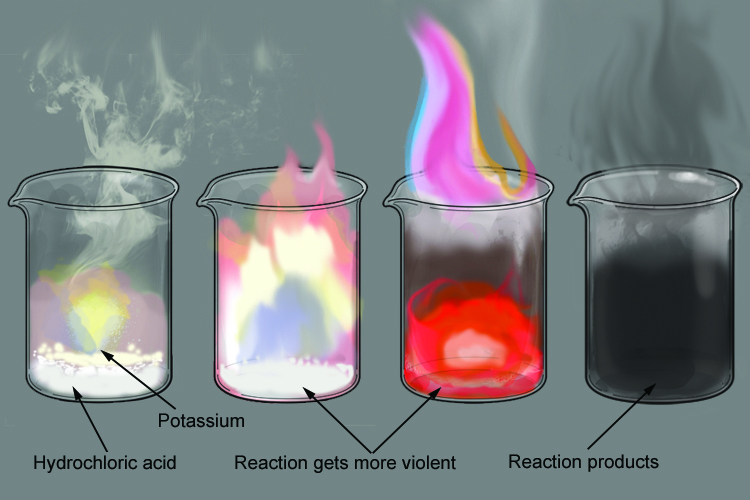
Does potassium react with dilute hydrochloric acid. Carbonate minerals such as calcite tend to fizz or efferves when tested with Hydrochloric Acid HCl. The potassium immediately ignites on contact with the acid producing a bright lilac flame that quickly.
Metals to the left of hydrogen in the electrochemical series react with hydrochloric acid. You can see the atomic emiss. This results because potassium chloride is combined with sulfuric.
How does sodium react with acid. Reaction to Hydrochloric Acid HCl Click here to go back to the main page. Click to see full answer.
It reacts with the fumes of nitric acid at 15 C 59 F to form sodium nitrate and with acetic and sulfuric acids to form sodium acetate and sodium sulfate. Metals to the right of hydrogen in the electrochemical series such as copper silver and gold do not react. These elements include lithium potassium calcium sodium magnesium aluminum zinc iron and lead.
The sodium hydroxide reacts with hydrogen ions removing them from the solution. Sodium is attacked by other strong mineral acids to form the corresponding salts. Hydrochloric acid evaporates off of potassium sulfate when its produced.
How does potassium sulfate react with hydrochloric acid. Potassium violently reacts with hydrochloric acid forming the potassium chloride and hydrogen gas. Potassium metal reacts with hydrochloric acid in this video.
The reaction between potassium and hydrochloric acid is over very quickly. Heating small pieces of Potassium in air results in the substance melting without any flame being seen and turning instantly into a mixture of potassium peroxide and potassium super oxide. KOHaq HClaq rarr KClaq H_2Ol And at the equivalence point the pH7 ie.
Strong acid and strong base gives a NEUTRAL solution. The reaction is very quick due to potassiums position in the reactivity series although the speed of the reaction can also be affected by the strength of the acid. As hydrochloric acid is added to the potassium chromate solution the yellow colour turns to orange.
Metals to the left of hydrogen in the. About 5 mL of the HCl is added to a small piece of potassium metal.
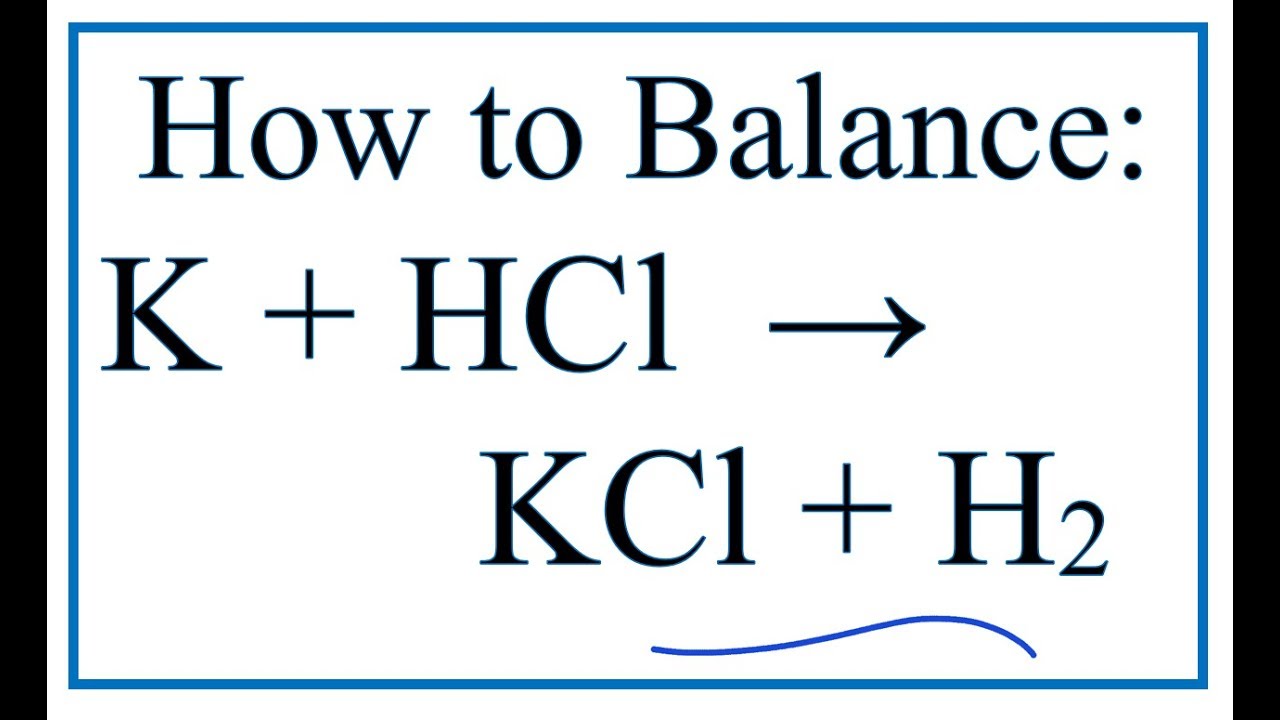
How To Balance K Hcl Kcl H2 Potassium Hydrochloric Acid Dilute Youtube

Reaction Of Hcl Aq With A Lithium B Sodium And C Potassium A Download Scientific Diagram

Potassium Metal Reacting With Concentrated Hydrochloric Acid Youtube
0 Comments